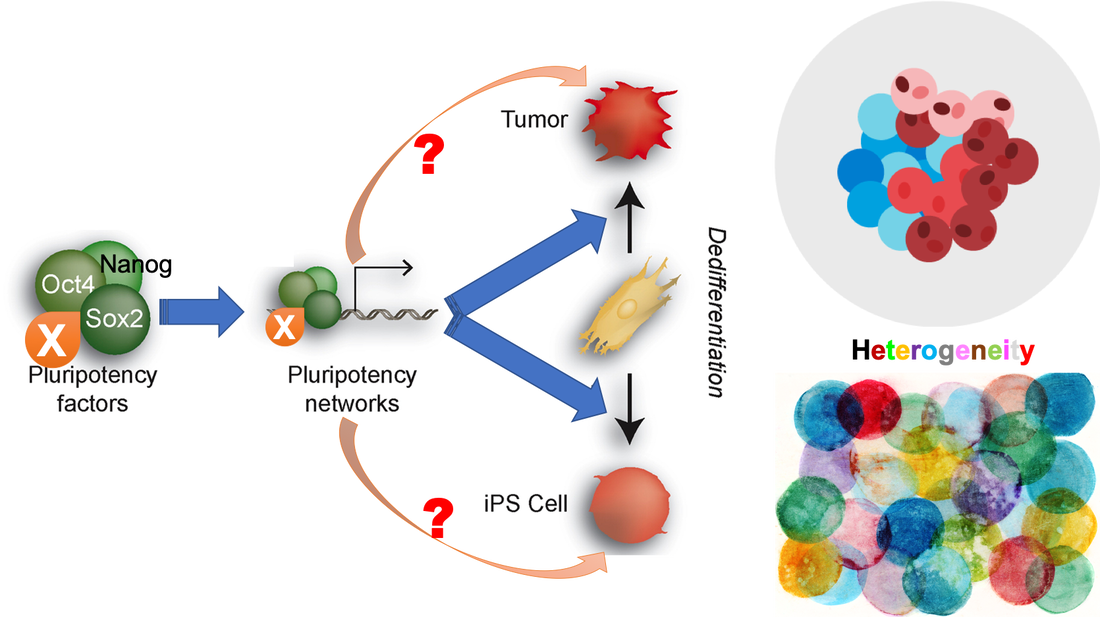
Many pluripotency factors, including the master transcription factors Oct4, Sox2 and Nanog, and signaling pathways (e.g., β-catenin signaling), are also operative in cancer cells (see Figure above). Such an intrinsic connection between pluripotency and tumorigenesis is further supported by recognized roles of additional pluripotency-associated factors (e.g., ZFP281, NAC1, PSPC1 and NONO) and epigenetic regulators (e.g., TET1/2) in cancer. We have begun to dissect pluripotency regulators and signaling pathways in cancer development (see our publications). For example, we have studied functions of the BTB-POZ transcription factor NAC1, initially discovered in our Nanog interactome studies (Wang et al., Nature 2006), in human ovarian (SKOV3) and cervical (HeLa) cancer cells (Paper-1; Paper-2). We have also identified novel Tet2 partners in both ESCs (Guallar et al., Nature Genetics 2018) and hematopoietic cells, the latter of which led to a better understanding of hypermutagenicity of HSPCs in Tet2 mutant hematopoietic cells and animals (Pan et al., Nature Communications 2017).
Accordingly, a new direction of our lab research is to employ our well-established proteomic and genomic approaches together with the rich resources of information and reagents from our stem cell studies to facilitate understanding of the stemness in cancers. Owing to the heterogeneous nature of both cancer/tumor cells and ESCs/iPSCs, we are particularly interested in studying how the stemness factors we have identified in pluripotent stem cells may also play roles in contributing to the stemness in cancer. The goal is to employ the ESC/iPSC platform for discovery and dissection of stemness factors and molecular pathways that may operate in tumors, providing novel targets for cancer intervention and treatment.
Accordingly, a new direction of our lab research is to employ our well-established proteomic and genomic approaches together with the rich resources of information and reagents from our stem cell studies to facilitate understanding of the stemness in cancers. Owing to the heterogeneous nature of both cancer/tumor cells and ESCs/iPSCs, we are particularly interested in studying how the stemness factors we have identified in pluripotent stem cells may also play roles in contributing to the stemness in cancer. The goal is to employ the ESC/iPSC platform for discovery and dissection of stemness factors and molecular pathways that may operate in tumors, providing novel targets for cancer intervention and treatment.