Vital cellular functions of pluripotent stem cells require the coordinated action of a large number of proteins that assemble into an array of multi-protein complexes of distinct composition and structure (protein-protein interactions). In addition, physical interactions between regulatory pluripotency transcription factors and their target genes (protein-DNA interactions) provide insights into differential gene expression dictating the pluripotency program. Analysis of the transcriptional regulatory complexes encompassing intricate protein-protein and protein-DNA interactions is key to understanding maintenance and establishment of stem cell pluripotency.
Our research focuses on pluripotency are in the following areas:
Our research focuses on pluripotency are in the following areas:
- Transcriptional and epigenetic regulation of stem cell pluripotency.
- RNA binding proteins (RBPs) in pluripotency and reprogramming control.
- DNA/RNA methylation (5mC/m5C) in pluripotency and reprogramming control.
- Translational control of stem cell pluripotency.
1. Pluripotency Interactome Studies
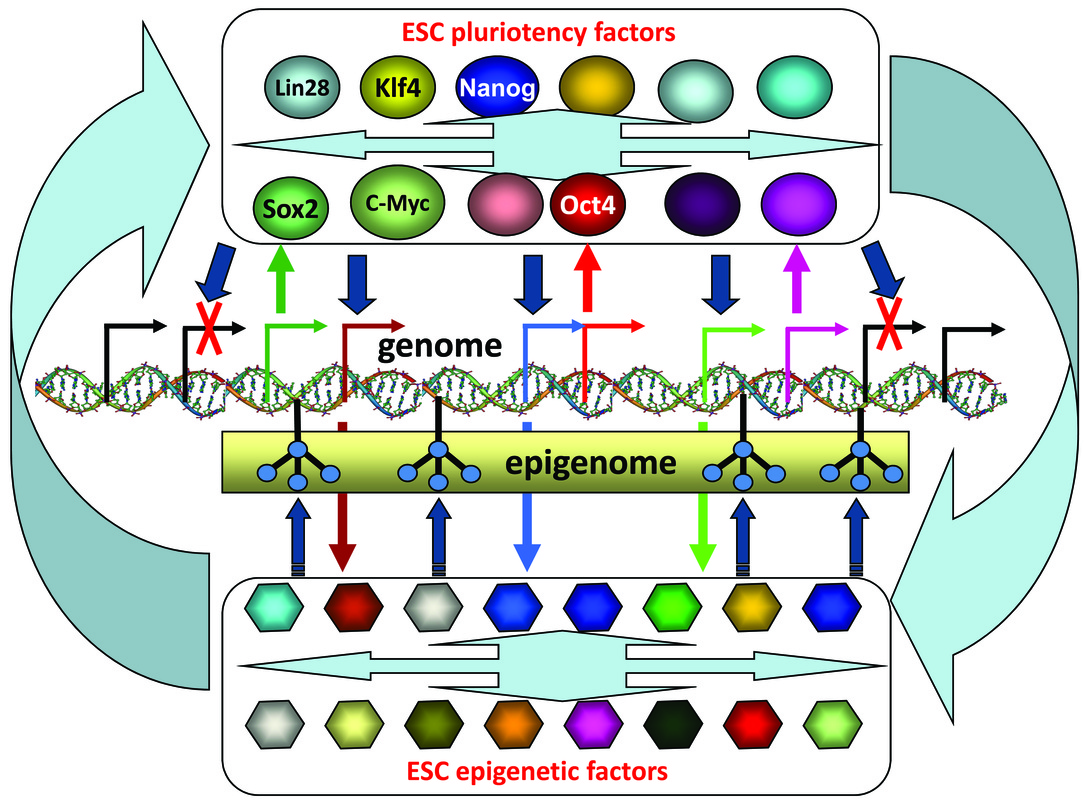
Pluripotency, the ability of a cell to give rise to all types of cells of an organism, is a fundamental characteristic of embryonic stem cells (ESCs). The basis of pluripotency resides in conserved regulatory networks and protein interaction networks of numerous transcription factors (TFs) and epigenetic regulators, which act together to repress developmental genes and activate stemness genes in ESCs (Figure 1). Oct4, Sox2 and Nanog are key components of the core regulatory network that governs ESC pluripotency. Epigenetic regulators, such as Polycomb group (PcG) proteins, SWI/SNF proteins and Mi-2/NuRD complex proteins, are largely involved in executing pluripotency (i.e., proper differentiation of ESCs). Understanding the interconnections and interactions among these pluripotency TFs and epigenetic cofactors is critical for maintaining and differentiating pluripotent stem cells for therapeutic application.
Pluripotency, the ability of a cell to give rise to all types of cells of an organism, is a fundamental characteristic of embryonic stem cells (ESCs). The basis of pluripotency resides in conserved regulatory networks and protein interaction networks of numerous transcription factors (TFs) and epigenetic regulators, which act together to repress developmental genes and activate stemness genes in ESCs (Figure 1). Oct4, Sox2 and Nanog are key components of the core regulatory network that governs ESC pluripotency. Epigenetic regulators, such as Polycomb group (PcG) proteins, SWI/SNF proteins and Mi-2/NuRD complex proteins, are largely involved in executing pluripotency (i.e., proper differentiation of ESCs). Understanding the interconnections and interactions among these pluripotency TFs and epigenetic cofactors is critical for maintaining and differentiating pluripotent stem cells for therapeutic application.
Figure 1. Interplay between genetic and epigenetic factors in ESCs. The cross-talk modes involve the reciprocal regulation of expression levels (small arrows) and direct physical interactions (big arrows).
2. Moleuclar Mechanisms of Pluripotency
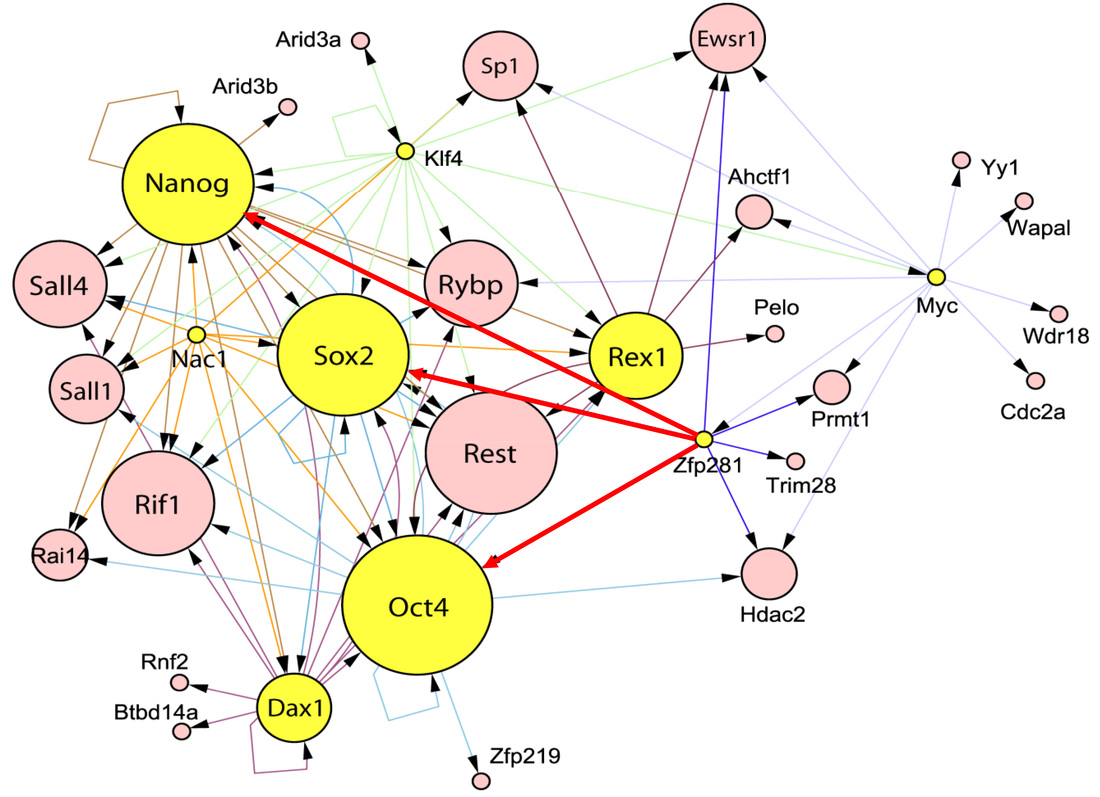
Following our pluripotency interactome (Wang et al., 2006) and transcriptional regulatory network (Kim et al., 2008) studies, we have constructed the functional regulatory network combining both protein-protein and protein-DNA networks and constructed the functional regulatory network controlling pluripotency of mouse ESCs (Figure 2). Our research has been focused on dissecting the molecular mechanisms underlying stem cell pluripotency by addressing the following questions:
Following our pluripotency interactome (Wang et al., 2006) and transcriptional regulatory network (Kim et al., 2008) studies, we have constructed the functional regulatory network combining both protein-protein and protein-DNA networks and constructed the functional regulatory network controlling pluripotency of mouse ESCs (Figure 2). Our research has been focused on dissecting the molecular mechanisms underlying stem cell pluripotency by addressing the following questions:
- How does Nanog molecule behave in the transcriptional complex for downstream target gene regulation? We discovered Nanog functions as a dimer in regulating stem cell pluripotency (Wang et al., PNAS 2008).
- How does ESC maintain an optimal level of Nanog for pluripotency? We discovered an Oct4-Sox2 independent, and Zfp281-dependent negative regulatory mechanism in fine-tuning Nanog expression for pluripotency (Fidalgo et al., Stem Cells 2011).
- How does Nanog function as a rheostat in controlling self-renewal and pluripotency of ESCs? We discovered a Zfp281/NuRD-mediated Nanog autorepression in controlling pluripotency and reprogramming (Fidalgo et al., PNAS 2012).
- How do other key Nanog/Oct4 partners contribute to establishment and maintenance of ESC pluripotency (projects ongoing)?
Figure 2. An integrated network controlling pluripotency of mouse ESCs. Key pluripotency factors and their protein interaction partners (Wang et al., Nature 2006) and DNA binding targets (Kim et al., Cell 2008) are integrated into a functional regulatory network as shown. The size of each circle reflects the degree of factor co-occupancy. Arrowhead indicates the direction of transcriptional regulation. Zfp281 is an upstream regulator of the three core pluripotency factors Nanog, Oct4 and Sox2.
3. RNA Binding Proteins and Alternative Splicing for Stem Cell Pluripotency
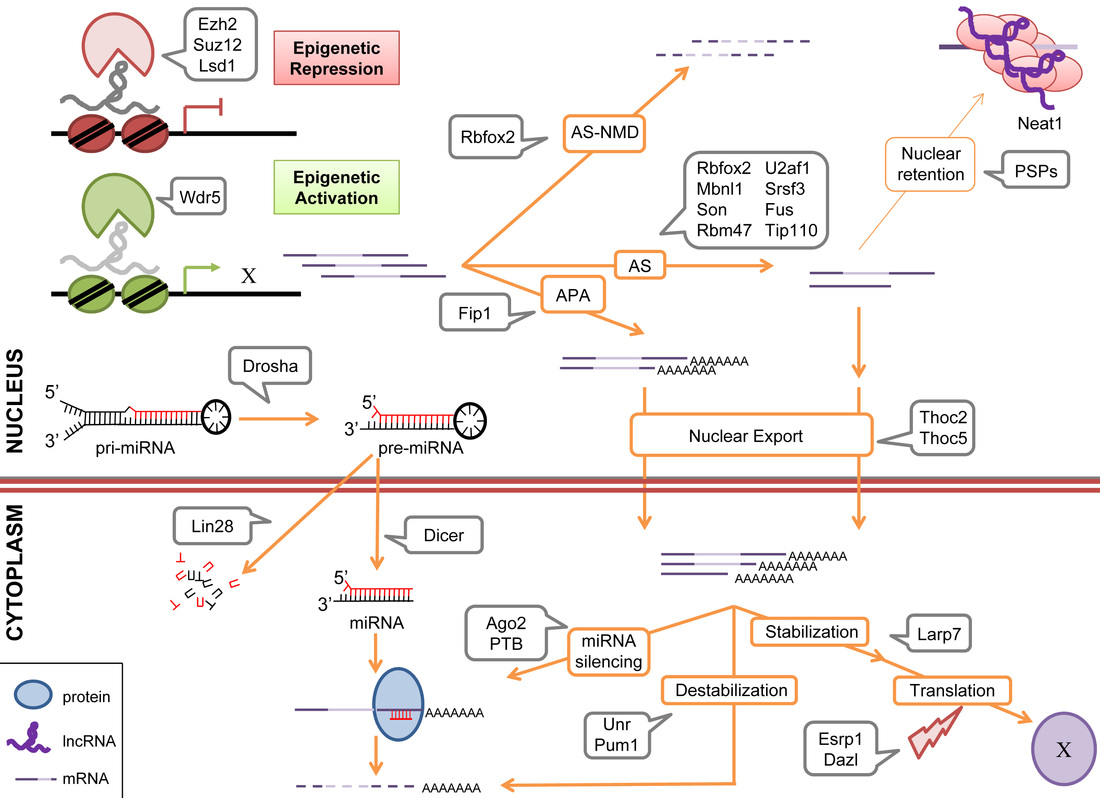
Embryonic stem cell maintenance, differentiation, and somatic cell reprogramming require the interplay of multiple pluripotency factors, epigenetic remodelers, and extracellular signaling pathways. RNA-binding proteins (RBPs) are involved in a wide range of regulatory pathways, from RNA metabolism to epigenetic modifications. In recent years we have witnessed more and more studies on the discovery of new RBPs and the assessment of their functions in a variety of biological systems, including stem cells. We summarized in Figure 3 on RBPs that have functional implications in pluripotency, differentiation, and/or reprogramming in both the human and mouse systems (Guallar and Wang, 2014).
We are interested in studying how RNA-binding proteins control alternative splicing of pluripotency associated genes in stem cell maintenance and during differentiation of ESCs toward to specific lineages.
Embryonic stem cell maintenance, differentiation, and somatic cell reprogramming require the interplay of multiple pluripotency factors, epigenetic remodelers, and extracellular signaling pathways. RNA-binding proteins (RBPs) are involved in a wide range of regulatory pathways, from RNA metabolism to epigenetic modifications. In recent years we have witnessed more and more studies on the discovery of new RBPs and the assessment of their functions in a variety of biological systems, including stem cells. We summarized in Figure 3 on RBPs that have functional implications in pluripotency, differentiation, and/or reprogramming in both the human and mouse systems (Guallar and Wang, 2014).
We are interested in studying how RNA-binding proteins control alternative splicing of pluripotency associated genes in stem cell maintenance and during differentiation of ESCs toward to specific lineages.
Figure 3. Summary of RNA-Binding Proteins (RBPs) in Pluripotency Control. RBPs with important roles in ESC maintenance and/or differentiation are depicted according to the RNA metabolism step in which they are implicated. Ezh2, Suz12 and Lsd1 repress transcription whereas Wdr5 activates it, binding to chromatin through lncRNAs. Rbfox2 is implicated both in AS and AS-NMD, wherease Mbnl1, Son, Rbm47, U2af1, Srsf3 and Fus have only been reported to regulate AS. Fip1 mediates APA. Once processed, transcripts can either be retained in paraspeckles by PSPs or exported to the cytoplasm. Thoc2 and Thoc5 regulate the transport of important pluripotency-related transcripts in ESC. In the cytoplasm, transcripts are stabilized and transcribed to proteins or targeted for destabilization and/or miRNA silencing by Unr and Pum1 or Ago2, respectively. Translation efficiency can be also subjected to regulation, through proteins such as Larp7 (stabilizer) or Esrp1 and Dazl (reduce ribosomal loading). Processing of pri-miRNAs by Drosha and pre-miRNA by Dicer gives rise to mature miRNAs available in the cytoplasm for transcript silencing. Lin28 affects its targets through targeting pre-miRNAs for degradation, therefore reducing the steady-state levels of miRNAs. From top to bottom: AS-NMD: alternative splicing-coupled nonsense-mediated decay, AS: alternative splicing; APA: alternative polyadenylation; PSPs: paraspeckle proteins; pri-miRNA: primary microRNA transcript; pre-miRNA: precursor microRNA transcript; miRNA: microRNA. (Guallar and Wang, 2014).
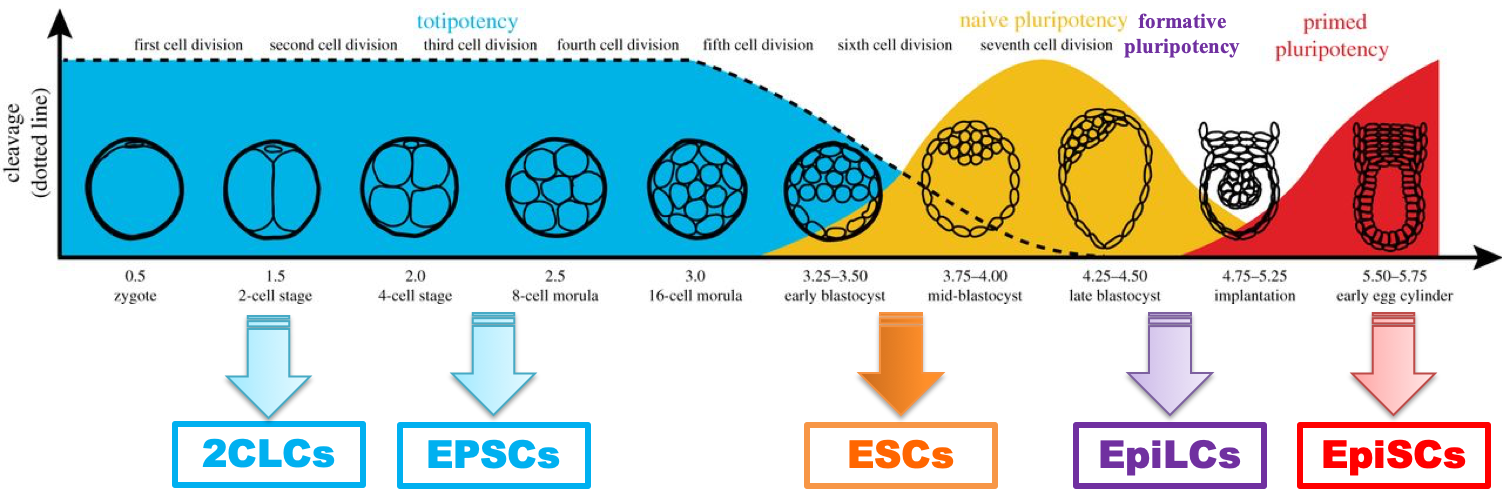
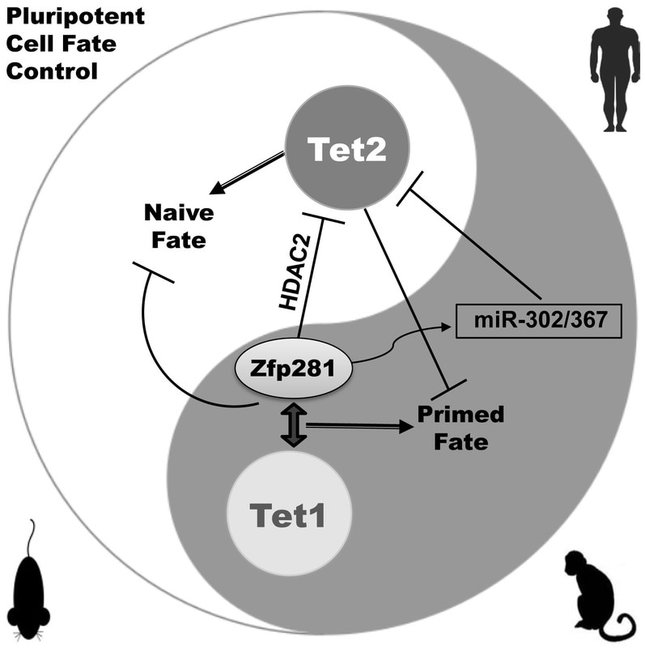
4. Distinct Pluripotency States: Naive versus Primed Pluripotency
We are employing genomic and proteomic approaches to dissect the genetic and epigenetic machineries demarcating the naive and primed pluripotency states in mouse ESCs and EpiSCs, respectively, and also in human ESCs cultured under naive and primed conditions, respectively.
We are employing genomic and proteomic approaches to dissect the genetic and epigenetic machineries demarcating the naive and primed pluripotency states in mouse ESCs and EpiSCs, respectively, and also in human ESCs cultured under naive and primed conditions, respectively.
Figure 4. An evolutionarily conserved pluripotent cell fate (PCF) gene signature functionally distinguishes naive from primed cells. An RNAi screen for PCF gene regulators led to the discovery of opposing functions of Tet1 and Tet2 mediated by Zfp281 in transcriptional and post transcriptional control of alternative pluripotent states (Fidalgo et al., Cell Stem Cell 2016).