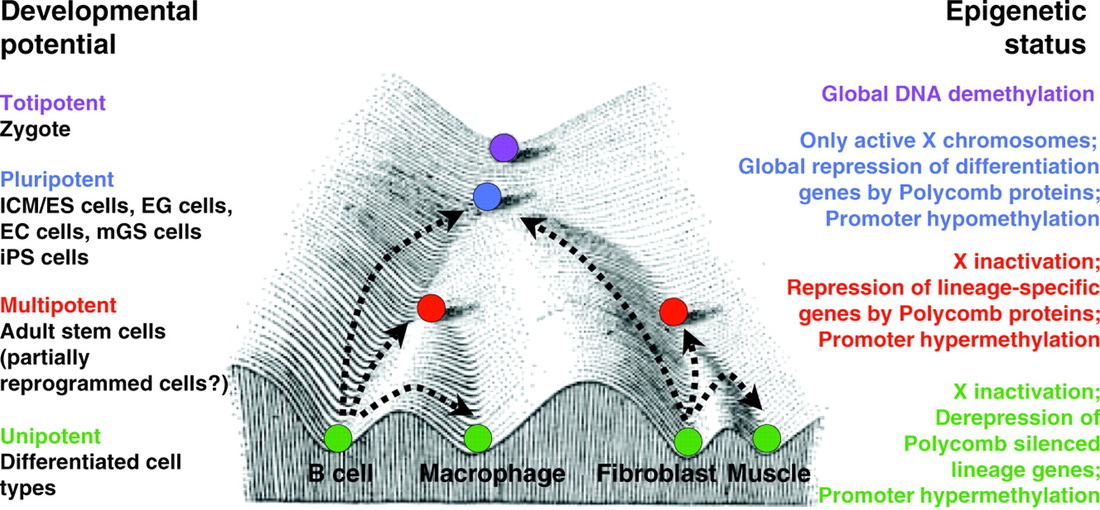
Totipotency is the ability of a single cell to divide and produce all of the differentiated cells in an organism. zygotes are prototype examples of totipotent cells. In the spectrum of cell potency, totipotency represents the cell with the greatest differentiation potential and sits atop of the Conrad Waddington epigenetic landscape (Figure 1).
Figure 1. Stem Cell Potency and Epigenetic Status (illustration from Development 2009;136:509-523).
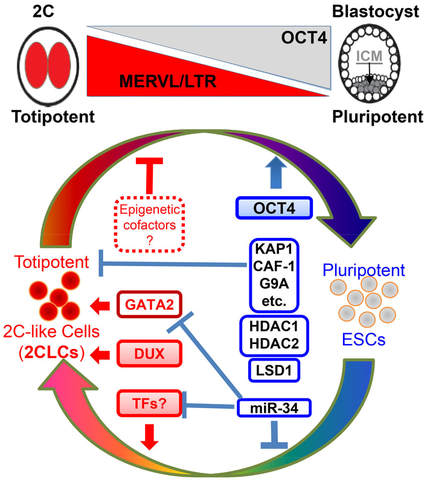
We are interested in identifying key molecules that play positive or negative roles in regulating the totipotent state and understanding the differential epigenetic makeups between pluripotency and totipotency (Figure 2).
Figure 2. A Putative Model of Molecular Control of Totipotency and Pluripotency. Factors and/or noncoding molecules from published studies were shown. More studies were conducted so far on understanding pluripotency establishment and maintenance (blue/right side). What are other coding and non-coding molecules than DUX and GATA2 that control active establishment and maintenance of totipotency remains to be identified.
Understanding how MERVL is dynamically controlled in pluripotent stem cells will be instrumental to the mechanistic dissection of molecular mechanisms underlying totipotency. Recently we have discovered that MERVL expression is under both transcriptional and post-transcriptional regulation through the HDAC1/2 corepressor and RNA-binding protein PSPC1-recruited TET2 complexes, respectively (Figure 3).
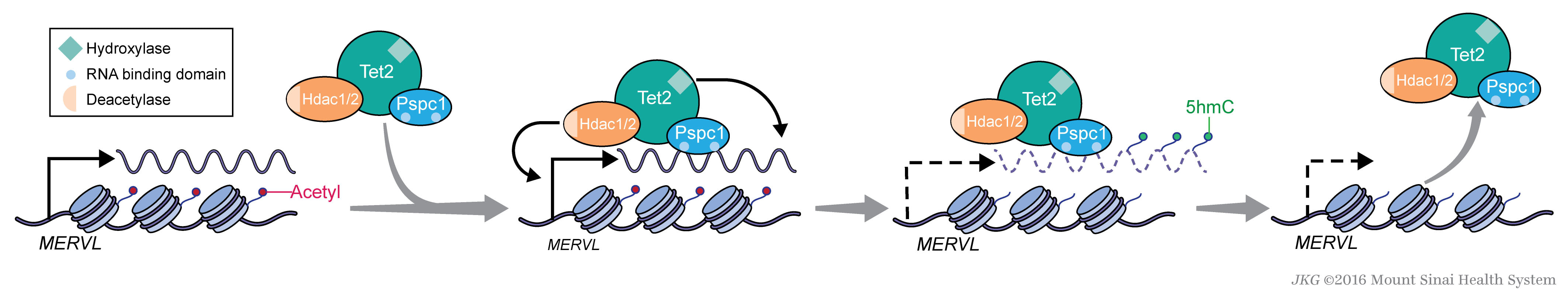
Figure 3. A Model of MERVL Regulation in ESCs. Fluctuating expression of MERVL in ESCs is partly controlled by TET2-mediated RNA hydroxymethylation and destabilization of MERVL transcripts (Guallar et al., 2018).
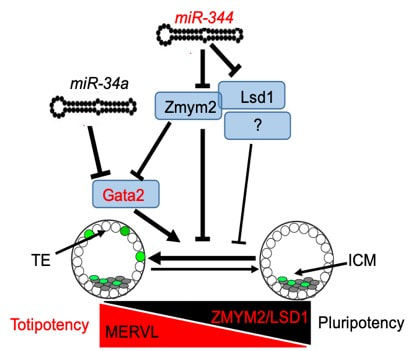
Figure 4. DUX-miR-344-ZMYM2-mediated activation of MERVL LTRs induces a totipotent 2C-like state.
Mouse embryonic stem cells (ESCs) sporadically express preimplantation two-cell-stage (2C) transcripts, including MERVL endogenous retrovirus and Zscan4 cluster genes. Such 2C-like cells (2CLCs) can contribute to both embryonic and extraembryonic tissues when reintroduced into early embryos, although the molecular mechanism underlying such an expanded 2CLC potency remains elusive. We examine global nucleosome occupancy and gene expression in 2CLCs and identified miR-344 as the noncoding molecule that positively controls 2CLC potency. We find that activation of endogenous MERVL or miR-344-2 alone is sufficient to induce 2CLCs with activation of 2C genes and an expanded potency. Mechanistically, miR-344 is activated by DUX and post-transcriptionally represses ZMYM2 and its partner LSD1, and ZMYM2 recruits LSD1/HDAC corepressor complex to MERVL LTR for transcriptional repression. Consistently, zygotic depletion of Zmym2 compromises the totipotency-to-pluripotency transition during early development. Our studies establish the previously unappreciated DUX-miR-344-Zmym2/Lsd1 axis that controls MERVL for expanded stem cell potency (Yang et al. Cell Stem Cell 2020).
Mouse embryonic stem cells (ESCs) sporadically express preimplantation two-cell-stage (2C) transcripts, including MERVL endogenous retrovirus and Zscan4 cluster genes. Such 2C-like cells (2CLCs) can contribute to both embryonic and extraembryonic tissues when reintroduced into early embryos, although the molecular mechanism underlying such an expanded 2CLC potency remains elusive. We examine global nucleosome occupancy and gene expression in 2CLCs and identified miR-344 as the noncoding molecule that positively controls 2CLC potency. We find that activation of endogenous MERVL or miR-344-2 alone is sufficient to induce 2CLCs with activation of 2C genes and an expanded potency. Mechanistically, miR-344 is activated by DUX and post-transcriptionally represses ZMYM2 and its partner LSD1, and ZMYM2 recruits LSD1/HDAC corepressor complex to MERVL LTR for transcriptional repression. Consistently, zygotic depletion of Zmym2 compromises the totipotency-to-pluripotency transition during early development. Our studies establish the previously unappreciated DUX-miR-344-Zmym2/Lsd1 axis that controls MERVL for expanded stem cell potency (Yang et al. Cell Stem Cell 2020).